- Chronic toxicity study (1, 3, 6, 9 or 12 months) and study of cumulative effect on mature animal are performed according to the Manual for conducting preclinical studies of drugs. Under the editorship of Mironov A.N., Bunatyan N.D. et al., M., publishing house “Grif and K”, 2012; EPA Health Effects Test Guidelines, OPPTS 870.4100 Chronic Toxicity, ICH – S4.
- Chronic toxicity study on non-mature animals is carried out according to the Manual for conducting preclinical studies of drugs. Under the editorship of Mironov A.N., Bunatyan N.D. et al., M., publishing house “Grif and K”, 2012 and FDA Nonclinical Safety Evaluation of Pediatric Drug Products.
- Chronic toxicity study of pharmacological substances on rabbits with an assessment of local irritant effect is performed according to the Manual for conducting preclinical studies of drugs. Under the editorship of Mironov A.N., Bunatyan N.D. et al., M., publishing house “Grif and K”, 2012.
- Chronic toxicity study is also conducted according to OECD protocols on testing of chemicals:
| 407 | Repeated dose 28-day oral toxicity in rodents |
| 408 | Repeated dose 90-day oral toxicity in rodents |
| 409 | Repeated dose 90-day oral toxicity in non-rodents |
| 410 | Repeated Dose Dermal Toxicity: 21/28-day Study |
| 419 | Delayed neurotoxicity of Organophosphorus Substances: 28-day repeateddose study |
| 452 | Chronic toxicity studies |
| 453 | Combined chronic toxicity/cancinogenicity studies |
Chronic toxicity study can be performed in rodents (mice, rats, hamsters, guinea pigs) and non- rodents (rabbits and mini pigs)
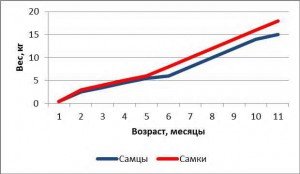
Weight of mini pigs Bishtrasser Knaus
Characteristics:
Birth Weight: 0.45 kg
Age at weaning: 4 weeks (3 kg)
Sexual maturity of males: 3-4 months (7-9 kg)
Sexual maturity of females: 4-5 months (8-12 kg)
Number of offspring: 5-8
Termination of weight gain: 18-22 months